Why this study – the rationale/objective?
Acute decompensated heart failure (ADHF) is a major cause of hospitalisation, often associated with persistent volume overload despite the use of loop diuretics. Acetazolamide, a carbonic anhydrase inhibitor acting at the proximal tubule, may potentiate the effect of loop diuretics by promoting natriuresis. In the ADVOR study, Professor Wilfried Mullens (Oost-Limburg Hospital, Genk, Belgium) and colleagues investigated whether adding acetazolamide to intravenous loop diuretics could enhance decongestion in patients with ADHF.
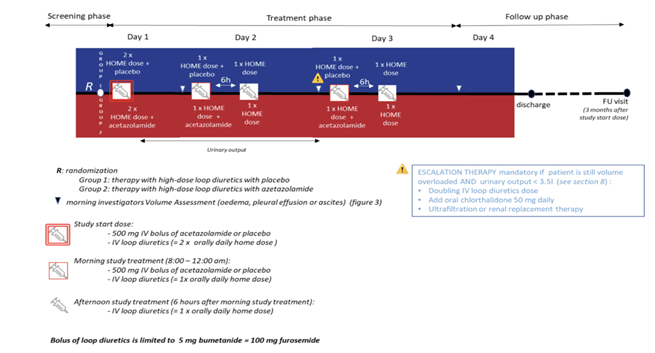
Diuretic protocole - Source: N Engl J Med 2022;387:1185-95. DOI: 10.1056/NEJMoa2203094
How was it executed – the methodology?
The ADVOR trial is a multicenter, randomised, double-blind, placebo-controlled study, supported by the Belgian Health Care Knowledge Center under the KCE Trials Program (KCE-17001) and it is conducted at 27 centers in Belgium. Hospitalised patients with ADHF showing clinical signs of volume overload (i.e., edema, pleural effusion, or ascites), and an N-terminal pro–B-type natriuretic peptide level of more than 1000 pg per milliliter or a B-type natriuretic peptide level of more than 250 pg per milliliter and receiving a stable dose of loop diuretics (≥40 mg of furosemide per day) were included.
The exclusion criteria for the study were:
- Patients receiving maintenance therapy with acetazolamide or any other proximal tubular diuretic, including sodium–glucose cotransporter 2 (SGLT2) inhibitors.
- A systolic blood pressure is less than 90 mm Hg.
- And an eGFR less than 20 mL/min/1.73 m².
They were randomised to receive either 500 mg of intravenous acetazolamide once daily or a placebo, in addition to a doubled dose of their usual intravenous loop diuretic. Treatment continued for 3 days or until complete decongestion was achieved.
A timed urine collection began after bladder emptying and the first dose of loop diuretics, lasting 30–48 hours. If urine output was under 3.5 liters and fluid overload remained, treatment was intensified. A daily congestion score (0–10), based on edema, pleural effusion, and ascites, was recorded before morning diuretics, at discharge, and during 3-month follow-up. The primary endpoint was successful decongestion within 3 days of randomization.
The main secondary endpoints were the composite outcome of all-cause mortality or heart failure rehospitalisation during the 3-month follow-up and the length of stay at the reference hospital.
What is the main result?
A total of 519 patients were randomised to receive either acetazolamide (259 patients) or placebo (260 patients) at 27 sites in Belgium. Successful decongestion was observed in 108 of 256 patients (42.2%)in the acetazolamide group (3 patients did not receive the assigned acetazolamide) and in 79 of 259 patients (30.5%) in the placebo group (1 patient did not receive the placebo) (risk ratio, 1.46; 95% confidence interval [CI], 1.17 to 1.82; p < 0.001).
The effect of acetazolamide on the primary outcome was generally consistent across subgroups, though patients on higher doses of loop diuretics showed less benefit. Among those discharged alive, 78.8% in the acetazolamide group and 62.5% in the placebo group achieved successful decongestion (risk ratio 1.27; 95% CI, 1.13–1.43).
Death from any cause or rehospitalisation for heart failure occurred in 76 of 256 patients (29.7%) in the acetazolamide group and in 72 of 259 patients (27.8%) in the placebo group (risk ratio, 1.07; 95% CI, 0.78 to 1.48).
Treatment with acetazolamide was associated with a higher cumulative urine output and natriuresis, consistent with greater diuretic efficacy. The incidence of worsening renal function, hypokalemia, hypotension, and adverse events was similar between groups.
Critical reading and the relevance for clinical practice
The ADVOR trial provides strong evidence that adding acetazolamide to loop diuretics improves decongestion in patients hospitalised for ADHF. However, several points require careful consideration:
- Studied population: Because the trial was conducted exclusively in Belgium, nearly all participants were White, which may restrict the applicability and the generalisability of the results to other racial or ethnic groups. All patients in this study had chronic heart failure and were on long-term outpatient treatment with at least 40 mg of furosemide equivalent. Therefore, the findings may not apply to those with newly diagnosed heart failure.
- Renal function: The included patients had moderately impaired renal function (GFR > 20 ml/min/1.73 m²), which raises questions about the efficacy and safety of acetazolamide in patientswith more advanced renal impairment.
- Follow-up duration: The follow-up period was limited to 3 months, which does not allow for the assessment of long-term effects of adding acetazolamide on mortality, rehospitalizations, or quality of life.
- Comparison with other approaches: The study did not compare acetazolamide with other strategies for optimizing diuresis, such as the addition of thiazide diuretics. Use of sodium–glucose co-transporter 2 (SGLT2) inhibitors— which were not yet standard of care at the study’s initiation—was an exclusion criterion. While SGLT2 inhibitors also act on the proximal tube, their mechanism of action differs, and their diuretic effect is considerably weaker. There is no reason to admit that the study’s findings would not be applicable to this population.
- Primary endpoint: The congestion score that was used for the assessment of the primary end point focused on the presence of edema in the lower limb, pleural effusion, and ascites — findings that are reflective of an assessment of mainly extracellular volume overload.
Since the study commenced in 2018 and extended through 2022, patients received heart failure therapy in accordance with the 2017 and 2021 European Society of Cardiology (ESC) guidelines, as reported by the authors. The pharmacological regimen included beta-blockers, angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), angiotensin receptor-neprilysin inhibitors (ARNIs), and mineralocorticoid receptor antagonists (MRAs), in addition to loop diuretics.
Sacubitril/valsartan was introduced into clinical practice in 2016, while gliflozins—sodium-glucose co-transporter 2 (SGLT2) inhibitors—were incorporated into the therapeutic arsenal in 2021.
According to current guidelines, ACE inhibitors or sacubitril/valsartan, beta-blockers, MRAs, and gliflozins are recommended as first-line therapy for all patients with heart failure, given their demonstrated efficacy in reducing all-cause mortality and heart failure-related hospitalisations (Class I recommendation).
In the present study, the authors did not report the concurrent use of SGLT2 inhibitors, nor did they provide a detailed breakdown of individual treatment regimens, likely due to adherence to two differing sets of guidelines during the study period.
It is noteworthy that, in contrast to the 2016 sequential treatment approach—where patients initiated therapy with beta-blockers and ACE inhibitors, followed by the addition of an MRA after three months if the left ventricular ejection fraction (LVEF) remained below 35%—the updated guidelines now advocate for the simultaneous initiation of all four drug classes at low doses, with gradual up-titration (a combination therapy strategy).
Furthermore, with respect to treatment-related adverse effects—particularly renal impairment—the authors did not report detailed creatinine measurements during the three-month follow-up period.
Conclusion
Adding acetazolamide to loop diuretics appears to be a promising therapeutic option for improving decongestion in patients hospitalized for ADHF. However, further studies are needed to confirm these findings in more diverse populations, assess long-term effects, and compare this approach with other therapeutic strategies.
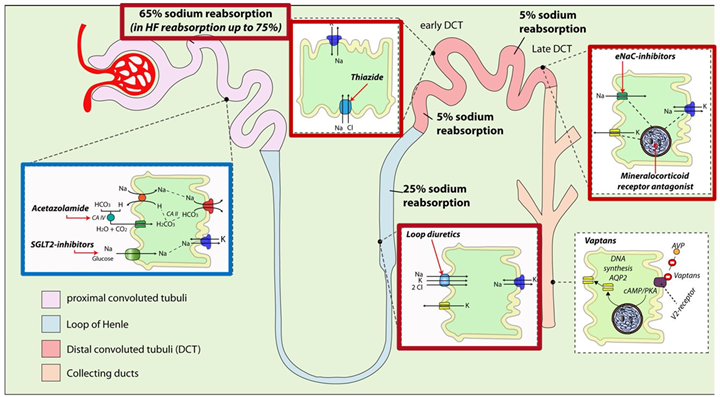
Sites, mode of action and effects on sodium reabsorption in the nephron of different diuretics - Source: Mullens et al EUR j HF 2019; 21:137 DOI: 10.1002/ejhf.1369






