Why this study – the rationale/objective?
The benefits of SGLT2 inhibitors in chronic heart failure (HF) are well established, including reductions in major outcomes and improvements in diuresis, remodelling, and symptoms.
In acute heart failure (AHF), evidence remains limited. In EMPA-RESPONSE-AHF (~80 patients), empagliflozin reduced the secondary composite outcome of in-hospital worsening HF, rehospitalisation, or death at 60 days, with increased urinary output and good tolerability. EMPULSE demonstrated that greater weight loss was linked to higher clinical benefit at 90 days, based on a hierarchical endpoint of all-cause mortality, HF events, and quality of life. DICTATE-AHF confirmed favourable diuretic profile and safety with early dapagliflozin initiation. A post-hoc analysis of SOLOIST-WHF suggested that initiation at or shortly before discharge—few days prior to discharge and thus likely still with signs of decompensation just in about one-quarter of patients— reduced mortality and HF events.
Building on these data, DAPA-ACT is the first large trial specifically testing dapagliflozin on hard outcomes and safety in AHF.
How was it executed – the methodology?
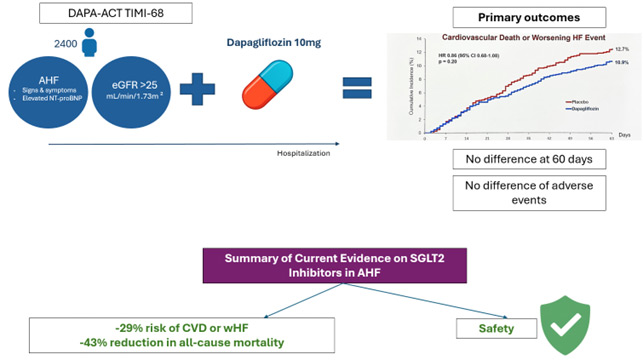
This was an investigator-initiated trial, sponsored by AstraZeneca, conducted between September 2020 and March 2025. It was a randomised, double-blind, multicentre study evaluating dapagliflozin 10 mg versus placebo in patients hospitalised with a primary diagnosis of HF, either de novo or worsening chronic HF.
Eligible patients were required to present with signs and symptoms of heart failure and elevated NT-proBNP, irrespective of left ventricular ejection fraction or diabetes status, and an eGFR greater than 25 mL/min/1.73 m². Randomisation occurred as early as possible, between 24 hours up to 14 days after hospital admission, in a 1:1 fashion.
The primary endpoint was a composite of cardiovascular death or worsening HF, the latter defined as hemodynamic deterioration, rehospitalisation for HF, or urgent outpatient visit for intravenous diuretic treatment. In addition, secondary analyses assessed the individual components of the primary endpoint, various combinations thereof, and all-cause mortality. Follow-up was set at 60 days.
The investigators also conducted a pre-specified meta-analysis of randomised trials on in-hospital initiation of SGLT2 inhibitors in AHF to provide a comprehensive evidence synthesis
What is the main result?
Overall, the trial enrolled approximately 2,400 patients with a mean age of 69 years; 34 % were women, 35 % had diabetes, and 45 % had an eGFR below 60 mL/min/1.73 m². About three-quarters of the population had a HF with reduced ejection fraction, and about half of the population had a de novo AHF.
The median NT-proBNP level at baseline was around 5,000 pg/mL. Randomisation occurred at a mean of 3.6 days after admission, with a total length of stay of approximately 5–7 days. Background guideline-directed medical therapy (GDMT) at admission was well represented, with 83 % of patients receiving beta-blockers, 70 % renin–angiotensin system inhibitors, and about half mineralocorticoid receptor antagonists.
The primary outcome occurred in 11 % of patients in the dapagliflozin group and 13 % in the placebo group. There was no statistically significant difference between groups, with a hazard ratio of 0.86 (95 % CI 0.68–1.08; p = 0.20). Secondary outcomes also showed no significant differences, and subgroup analyses were consistent across major clinical categories. Safety outcomes, including hypotension, worsening renal function, hypoglycemia, and diabetic ketoacidosis, were comparable between groups.
In the meta-analysis, SGLT2 inhibitors were associated with a 29 % lower risk of cardiovascular death or worsening HF (HR 0.71, 95 % CI 0.54–0.93; p = 0.012) and a 43 % reduction in all-cause mortality (HR 0.57, 95 % CI 0.41–0.80; p = 0.001).
Critical reading and the relevance for clinical practice
Although some may find the results somewhat discouraging, the main message of this study—that in-hospital initiation of dapagliflozin in patients with AHF did not significantly reduce the risk of cardiovascular death or HF events at 60 days—should not be misinterpreted. The authors themselves provide several plausible explanations for these findings, which at first glance may appear inconsistent with the established pleiotropic benefits of SGLT2 inhibitors.
First, the short follow-up period of only 60 days may have limited the ability to detect benefits on hard outcomes, which are well known over longer period. Second, the unexpectedly low event rates in both arms likely contributed to the lack of a detectable treatment effect, rendering the study underpowered for outcome differences.
These limitations should be acknowledged, but it is equally important to recognise the relatively low rates of background GDMT in the study population. Suboptimal implementation of life-saving therapies undoubtedly limits the overall clinical benefit patients can derive, irrespective of the study intervention. Moreover, real-world experience shows that once patients are discharged, initiation of new therapies becomes increasingly difficult, highlighting the importance of optimising treatment while still in hospital.
Therefore, despite the neutral results, the study should not dissuade clinicians from prescribing SGLT2 inhibitors in this population. While immediate initiation may not be strictly necessary, it is certainly advisable before hospital discharge. Early initiation is safe and may facilitate more effective diuretic management in the acute phase.
Central Figure. DAPA-ACT TIMI 68: Key Findings and Current Evidence on SGLT2i in AHF.
Abbreviations: AHF, acute heart failure; anti-proBNP, N-terminal pro-B-type natriuretic peptide; CI, confidence interval; CVD, cardiovascular death; HF, heart failure; HR, hazard ratio; eGFR, estimated glomerular filtration rate; SGLT2i, sodium–glucose cotransporter-2 inhibitors; WHF, worsening heart failure.
Authors: David D. Berg, MD, MPH; Siddharth M. Patel, MD, MPH; Paul M. Haller, MD, PhD; Abby L. Cange, BS; Michael G. Palazzolo, MS; Andrea Bellavia, PhD; Julia F. Kuder, MA; Akshay S. Desai, MD, MPH; Silvio E. Inzucchi, MD; John J.V. McMurray, MD; Eileen O’Meara, MD; Subodh Verma, MD, PhD; Jan Bělohlávek, MD, PhD; Jarosław Drożdż, MD, PhD; Béla Merkely, MD, PhD; Modele O. Ogunniyi, MD, MPH; Tomáš Drasnar, MD; Joseph L. Izzo, MD; Balazs Sarman, MD; John E. McGinty, MD; Krishnan Ramanathan, MB, ChB, FRACP, FRCPC; Angel J. Mulkay, MD; Andrzej Przybylski, MD, PhD; Christian T. Ruff, MD, MPH; Michelle L. O’Donoghue, MD, MPH; Sabina A.Murphy, MPH; Marc S. Sabatine, MD, MPH*; Stephen D. Wiviott, MD* for the DAPAACT HF-TIMI 68 Trial Committees and Investigators
Reference: doi.org/10.1161/CIRCULATIONAHA.125.076575





