Why this study – the rationale/objective?
Type 2 diabetes mellitus (T2DM) is a major contributor to both heart failure (HF) and chronic kidney disease (CKD). Microalbuminuria, assessed via urinary albumin-to-creatinine ratio (UACR), is a key marker of diabetic kidney injury and predictor of cardiorenal outcomes. Disease pathophysiology involves activation of the renin–angiotensin system (RAS) and overactivation of mineralocorticoid receptors—targeted respectively by ACEi/ARBs and MRAs. MRAs reduce albuminuria and slow CKD progression in patients with T2DM, but side effects and safety concerns have limited their use. Finerenone, a non-steroidal MRA, addresses these issues and has demonstrated cardiorenal benefits, leading to its recommendation in guidelines alongside GLP-1 receptor agonists and SGLT2 inhibitors, this latter also known to reduce albuminuria.
To date, clinical trials have primarily studied these therapies in placebo-controlled settings, and only secondary analyses have evaluated their effects on albuminuria. In addition, a key unanswered question is whether SGLT2 inhibitors and MRAs should be initiated sequentially or in combination. The CONFIDENCE trial was designed to address this knowledge gap, testing the efficacy and safety of combining finerenone with empagliflozin on albuminuria in patients with T2DM and CKD.
How was it executed – the methodology?
The trial was a randomized, double-blind, active-controlled study sponsored by Bayer and overseen by an independent data and safety monitoring board. It enrolled patients with T2DM, HbA1c <11%, eGFR 30–90 mL/min/1.73m² and severe albuminuria (UACR 100–5000 mg/g), all on stable ACEi/ARB therapy. Key exclusion criteria included type 1 diabetes, chronic symptomatic or recently worsened HFrEF, serum potassium >4.8 mmol/L or potassium binder use. Patients were randomized 1:1:1 to receive finerenone (20 mg or 10 mg if eGFR <60 mL/min/1.73m²), empagliflozin (10 mg) or combination therapy.
The study included seven visits over 210 days, with 180 days of treatment followed by a 30-day off-treatment phase. At each visit, blood and urine samples were collected (UACR was averaged across successive days), along with blood pressure measurements.
The primary efficacy outcome was UACR change at day 180. Secondary efficacy outcomes included UACR at 30 days post-discontinuation. Secondary safety outcomes included changes in eGFR, acute kidney injury (AKI), hyperkalemia, symptomatic hypotension, diabetic ketoacidosis, severe hypoglycemia and genital mycotic infections.
What is the main result?
A total of 800 patients were randomized, with balanced baseline characteristics: mean eGFR 54.2 ± 17.1 mL/min/1.73 m² (SD), median UACR 583 (292–1140) mg/g (IQR) and mean HbA1c 7.3 ± 1.2% (SD). At 3 months, combination therapy led to the greatest UACR reduction (LSM 0.48; 95% CI, 0.44–0.54), 29% and 32% greater than finerenone and empagliflozin alone, respectively (P<0.001). About 50% of patients in the combination group had >50% UACR reduction. At day 210, UACR increased after discontinuation, with an LSM ratio of 1.63 (95% CI, 1.49–1.78) in the combination group, greater than with either monotherapy.
Combination therapy was associated with a modest increase in potassium, a slight eGFR decline and greater BP reduction—all of which normalized after treatment cessation and were clinically non-significant. Adverse events were infrequent and comparable across groups, with <5% discontinuations and no major safety concerns.
Critical reading and the relevance for clinical practice
This trial addresses a clinically relevant question and provides solid evidence that combination therapy has an additive effect, using albuminuria as a surrogate of CVD outcomes and CKD progression. These findings are consistent with data from FIDELITY and CREDENCE trials.
The study is methodologically robust and the study population, though selected, reflects a clinical profile common in practice, enhancing generalizability. Although the cohort had well-controlled diabetes (mean HbA1c 7.3%), it's reasonable to expect that those with poorer control—and higher progression risk—could benefit equally or more from dual therapy. Including patients with severely increased albuminuria was appropriate, as this subgroup is at higher cardiorenal risk and may derive greater benefit. While this limits extrapolation to those with mild or moderate albuminuria, the findings remain relevant. The treatment effect likely reflects a true, biologically meaningful benefit, rather than a statistical artifact attributable to higher baseline albuminuria.
UACR is indeed a surrogate marker, but remains a strong endpoint—predictive of cardiorenal events, sensitive to early therapeutic change and easily monitored. Although subject to physiological variability (e.g., from sarcopenia or hydration), use of averaged samples improves reliability.
Though the trial did not assess hard endpoints like mortality or end-stage renal disease, the short follow-up is justified to capture early pharmacodynamic effects. Longer-term data will be key to confirm durability and clinical impact.
In conclusion, this trial supports an integrated approach to T2DM and CKD. The additive effect and favorable safety profile of combination therapy argue against stepwise escalation, favoring early, simultaneous use in appropriate patients.
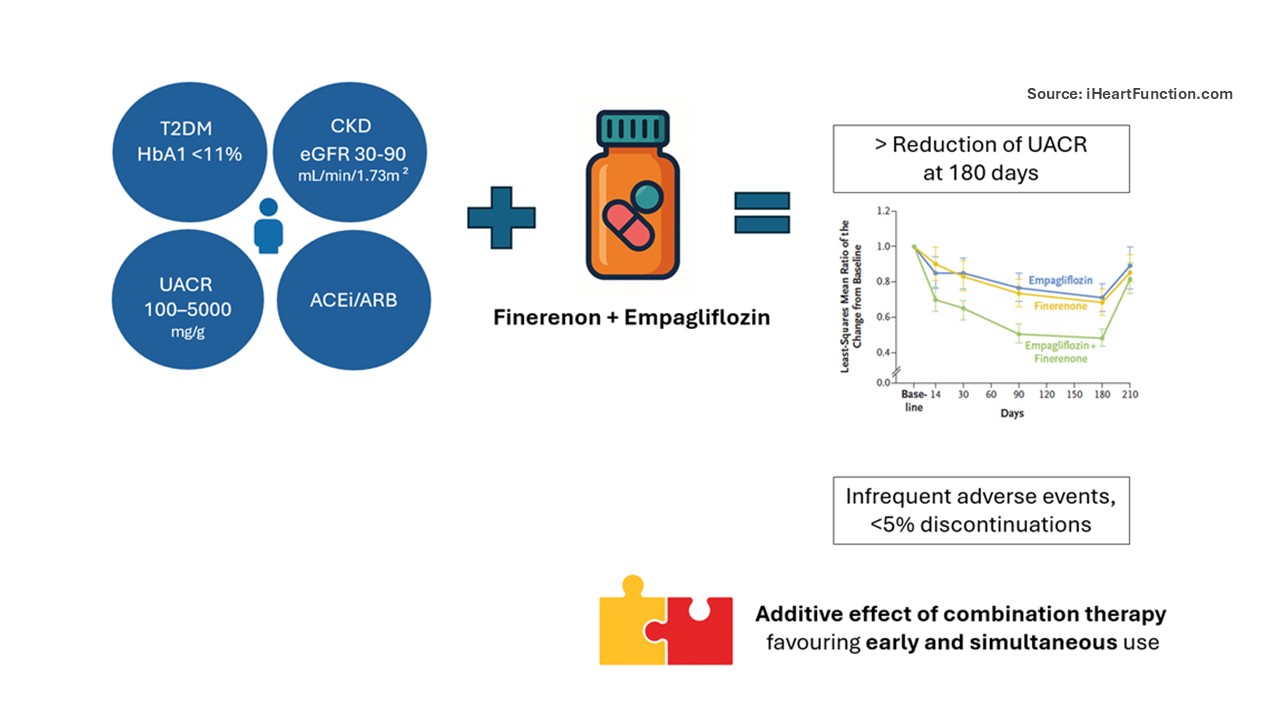
Designed by Jolie Bruno - Source iHeartFunction.com - The figure illustrates the main results of the study: in patients with type 2 diabetes and HbA1c <11%, UACR 100–500 mg/g, CKD stages 2–3b (eGFR 30–90 mL/min/1.73 m²), and background ACEi/ARB therapy, those randomized to the combination of finerenone and empagliflozin experienced a greater improvement in primary outcomes, i.e., greatest UACR reduction at 180 days, along with a low rate of adverse events. These findings support the additive value of combination therapy and favour its early and simultaneous initiation.





