Why this study – the rationale/objective?
The clinical paradigm that relies on a static measurement of left ventricular ejection fraction (LVEF) to categorize and manage heart failure (HF) is increasingly recognized as reductive. A single-point LVEF value offers only a snapshot, insufficient to capture the complex and dynamic progression of the disease. This fragmented view risks both underestimating reversible dysfunction and overlooking worsening trajectories. There is a growing need for a more precise approach—one that reflects the evolving nature of myocardial function and aligns more closely with patient outcomes and therapeutic need.
How was it executed – the methodology?
This document is the result of an expert consensus, synthesizing evidence from imaging studies, clinical registries, biomarker data and randomized trials. Consensus statements are designed to harmonize current knowledge and clinical practice when direct evidence is insufficient or fragmented. In this position paper, experts in HF provide a critical reinterpretation of how LVEF should be assessed and contextualized in modern HF care.
What is the main result?
Although the traditional LVEF-based classification continues to hold meaningful relevance due to its therapeutic implications, recent evidence has demonstrated that two of the so-called "fantastic four" drug classes—SGLT2 inhibitors and mineralocorticoid receptor antagonists (MRAs)—provide consistent benefit across the full spectrum of HF, regardless of LVEF. Consequently, once HF is diagnosed—based on clinical signs, symptoms and elevated natriuretic peptides—both agents, along with diuretics if congestion is present, should be initiated without delay, even in the absence of immediate LVEF availability. Nevertheless, timely assessment of LVEF remains crucial, as it guides the introduction of additional guideline-directed therapies, such as beta-blockers and RASi/ARNIs, and represents the foundational reference point upon which the patient’s clinical course is built.
The central and transformative concept proposed is a fundamental shift from static to dynamic assessment of LVEF, whereby trajectories of change—rather than isolated measurements—become the primary lens through which HF is classified for risk stratification, prognostication and treatment decision (Figure 1). This new paradigm recognizes three distinct phenotypes: HF with unchanged EF (HFuncEF), HF with improved EF (HFimpEF) and HF with worsening EF (HFwor). This trajectory-based framework establishes a pathophysiologically grounded basis for personalized approach to HF care.
Critical reading and the relevance for clinical practice
Scientific knowledge is constantly evolving, and with it, established clinical concepts may require revision. Questioning the conventional meaning and use of LVEF can initially create uncertainty. Yet, this position paper provides a powerful interpretative tool for understanding the clinical trajectory of patients with HF. Consider, for example, two patients with a current LVEF of 40%—one who declined from 60%, and another who improved from 20%. Despite sharing the same current value, their prognostic implications are clearly different. Both will likely receive the same foundational HF therapies, but the direction of LVEF change influences clinical judgment, follow-up strategies and diagnostic and therapeutic decisions.
Embracing a dynamic view of LVEF is not merely a semantic shift, but a change in substance. It enables a more comprehensive and individualized understanding of the disease process—beyond reliance on a single numerical value, which is subject to variability and can mislead clinicians and patients alike. Shifting from static thresholds to a longitudinal perspective—within a broader, multimodal assessment of cardiac structure and function—aligns with the principles of precision medicine.
However, this conceptual evolution must not result in clinical inertia. HF demands timely treatment, and optimal care should not depend on immediate access to all diagnostics. Evidence supports early initiation of SGLT2 inhibitors and MRAs across the full LVEF spectrum. These therapies should begin upon diagnosis—based on clinical signs and elevated natriuretic peptides—even before LVEF confirmation. This avoids unnecessary delays that could affect outcomes. Still, LVEF remains essential for implementing other cornerstone therapies reduced LVEF, including pharmacological treatments—such as beta-blockers and ARNIs—and device-based intervention, such as implantable cardioverter-defibrillators ICDs and cardiac resynchronization therapy (CRT), both linked to prognostic benefit.
As for device therapy, unnecessary postponement should be avoided. A three-month period is generally recommended before implantation, but if no improvement occurs with optimal medical therapy, the indicated device should be promptly provided. In some phenotypes, earlier intervention is warranted. For instance, in ischaemic cardiomyopathy, ICD indication may arise before the typical three-month evaluation period, while in highly arrhythmogenic forms, it may be warranted irrespective of LVEF evolution. Similarly, in patients with wide QRS and left bundle branch block, delaying cardiac resynchronization therapy may compromise benefit. Timely identification of these scenarios is essential to prevent under-treatment.
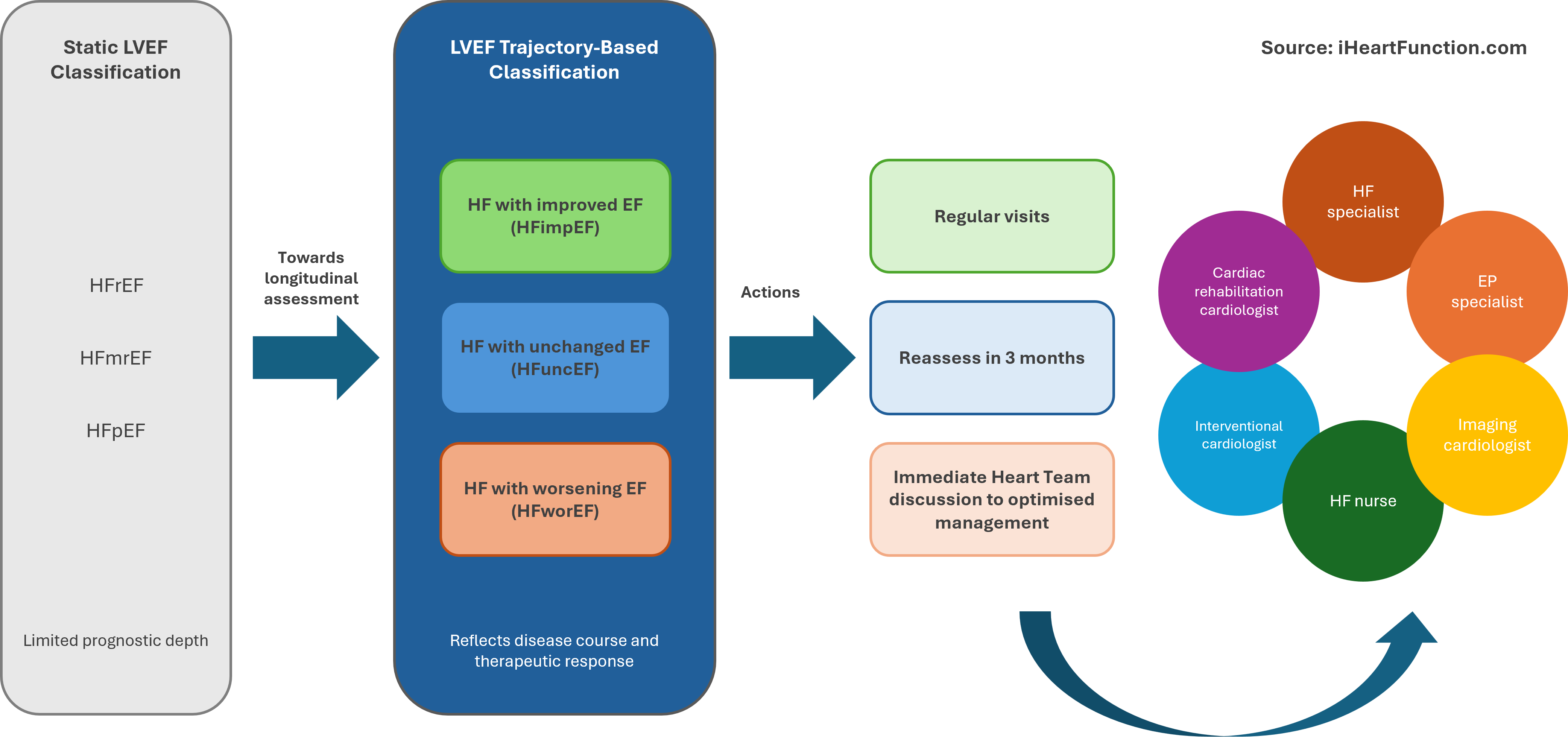
Designed by Jolie Bruno - Source iHeartFunction.com - LVEF trajectory-based classification and therapeutic implications: The figure illustrates the shift from a static LVEF classification, with limited prognostic information, to a trajectory-based approach, which better reflects disease progression and response to therapy. Each trajectory is associated with different clinical actions: patients with improved LVEF require regular follow-up, those with unchanged LVEF need early reassessment, along with optimization of therapy and all modifiable contributing factors. Patients with worsening LVEF require immediate multidisciplinary discussion in the Heart Team to optimize management.





