- A 58-year-old female nurse with no prior comorbidities, presented in November 2022 with progressive dyspnoea (NYHA class III–IV), orthopnea (requiring 3 pillows), dry nocturnal cough and paroxysmal nocturnal dyspnea
- No history of tobacco, alcohol, or substance use; no family history of cardiovascular disease.
- On examination she appeared ill; not pale, jaundiced, or edematous. BP 124/92 mmHg, HR 103 bpm, T 36.2°C, SpO₂ 96% (room air), Random blood glucose 5.9 mmol/L, BMI 21.3 kg/m². Laterally displaced heaving apex with S3 gallop and systolic murmurs; lungs were clear.
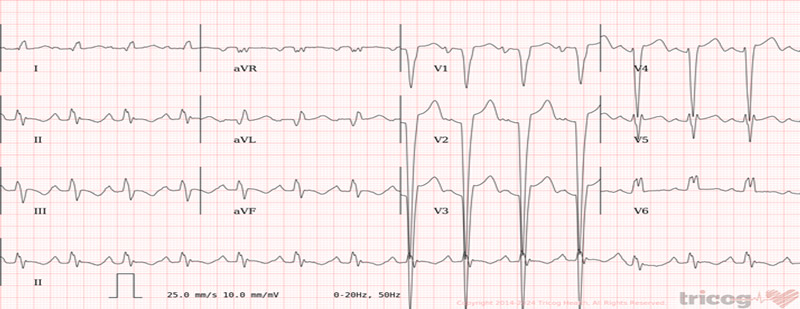
- ECG: sinus rhythm with Left Bundle Branch Block (LBBB), QRS 170 ms
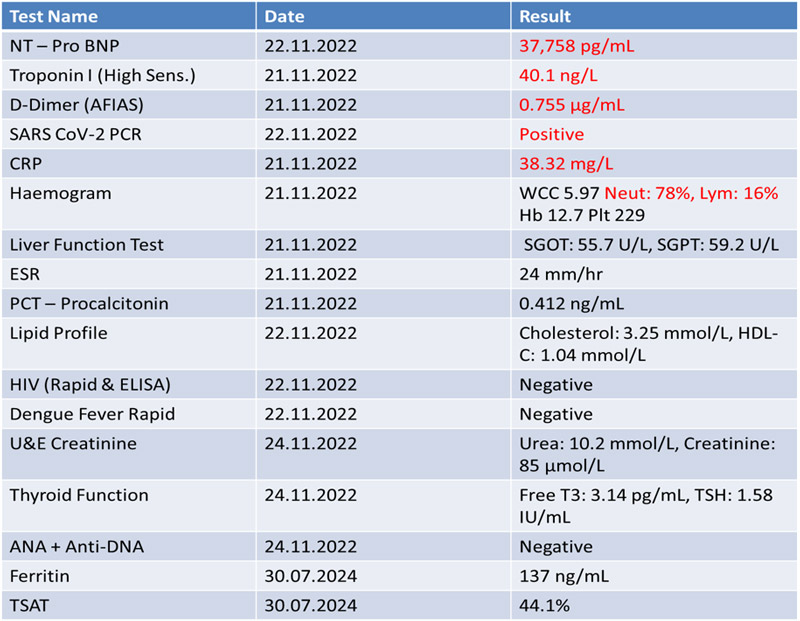
- Notable labs included an elevated NT-proBNP (37,758 pg/mL), troponin I (40.1 ng/L), and CRP (38.3 mg/L); SARS-CoV-2 PCR was positive.
- Echo: severe Dilated Cardiomyopathy (DCM) — LVEF 10–15%; LVEDd 81 mm; LVESd 78 mm; apical thrombus
- Chest CT: cardiomegaly, pericardial effusion, stage II pulmonary venous congestion; no COVID pneumonia
- IV diuretic: furosemide 80 mg at the time of referral, continued until clinical recompensation
- Guideline-directed medical therapy (GDMT) was initiated
- sacubitril/valsartan 12.5 mg BD
- empagliflozin 10 mg OD
- eplerenone 12.5 mg BD
- bisoprolol 1.25 mg BD (uptitrated to 2.5 mg mane and 1.25 mg nocte after 2 weeks)
- She also received:
- rivaroxaban 20 mg (remove torasemid!)
- supplemental oxygen
- SC enoxaparin 60 mg BD
- Supportive care: fluid balance monitoring and respiratory physiotherapy
(…) - A cardiology follow-up was scheduled for clinical revaluation, therapy uptitration and further diagnostic work-up with coronary angiography.
- NT-proBNP reduced from 37,758 pg/ml to 16,418 pg/ml two days post admission (56.5% reduction).
- Clinical improvement over 6 days of admission though BP remained low (83–90/60–65 mmHg).
- She was discharged on GDMT, torsemide 20mg od and rivaroxaban 20 mg OD (replaced enoxaparin).
- A cardiology follow-up was scheduled for coronary angiography.
- 3 weeks post discharge: Coronary angiography was performed and excluded obstructive coronary artery disease.
- 8th week review: Vitals: BP 90/59 mmHg, HR 63 bpm, SpO₂ 98%. No signs of congestion.
- Despite full adherence to GDMT (sacubitril/valsartan 12.5 mg BD, empagliflozin 10 mg OD, eplerenone 12.5 mg BD, bisoprolol 2.5 mg mane and 1.25 mg nocte) and torsemide 20mg od; she remained NYHA Class III with severe LV dysfunction (EF 15–20%) and persistent electrical dyssynchrony (QRS 170 ms).
- The persistently low blood pressure raised some concern about further GDMT uptitration.
Clinical presentation
Investigations
Inpatient management
Outpatient follow up
- Given the persistent HF symptoms, vital signs (which likely limited GDMT full uptitration), and the presence of LBBB with QRS 170 ms, CRT implantation was directly pursued
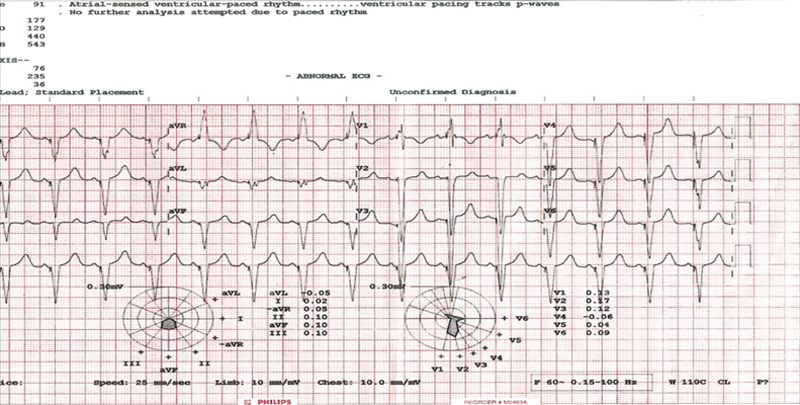
- A CRT-P selected over CRT-D due to financial constraints was implanted, with significant narrowing of QRS to 129ms
- The CRT device was programmed in DDD mode with a lower rate limit of 50 bpm, an upper tracking rate of 130 bpm, and an upper sensor rate of 120 bpm, maintaining biventricular pacing in 99.9% of cardiac cycles
- Because of cost constraints, no further investigations to assess the etiology of the cardiomyopathy were performed.
Outcome
Post-device implantation with GDMT unchanged, serial echo showed progressive LV reverse remodeling with improved EF, paralleled by stepwise gains in functional capacity on 6-minute walk tests as below:
Date | Interval post-CRT | LVESd (mm) | EF (%) by Simpson's biplane | 6MWT Distance | Predicted Distance (m) | % of Predicted |
|---|---|---|---|---|---|---|
31 Mar 2023 | ≈3 months | 76 | 15–20 | – | – | – |
23 Aug 2023 | ≈6 months | 50 | 35–40 | 400 m | 517 | 77% |
30 Oct 23 | ≈9 months | – | – | 450 m | 516 | 87% |
2 Apr 2024 | ≈14 months | 44 | 40–45 | – | – | – |
07 Janv 25 | ≈2 years | 35 | 55–60 | 415 m | 499.5 | 83% |
09 May 2025 | ≈2 years 3 months | – | – | 493.8 m | 502 | 98% |
Jul 25 | ≈2.5 years | 33 | 60–65 | 614m | 501 | 122% |
Chronological Vitals During Follow-up
GDMT was maintained unchanged; torsemide was decreased from 20 mg to 10 mg in June 2023 and kept at that dose thereafter.
Date | Interval post-CRT | BP (mmHg) | HR (bpm) | SpO₂ (%) |
|---|---|---|---|---|
31 Mar 23 | ≈3 months | 103/58 | 69 | 98 |
17 Jun 23 | ≈6 months | Lt 89/54; Rt 90/56 | 70 | 98 |
27 Mar 24 | ≈15 months | 96/64 | 71 | 98 |
07 Jun 24 | ≈18 months | 98/63 | 67 | 98 |
17 Jan 25 | ≈2 years | 102/67 | 62 | 97 |
14 Mar 25 | ≈2 years 3 months | 102/71 | 68 | 96 |
12 May 2025 | ≈ 2years 5 months | 93/66 | 71 | 96 |
12 Sept 25 | ≈ 2years 9months | 89/63 | 76 | 99 |
Echo: July 2025 (2.5 years post CRT-P)
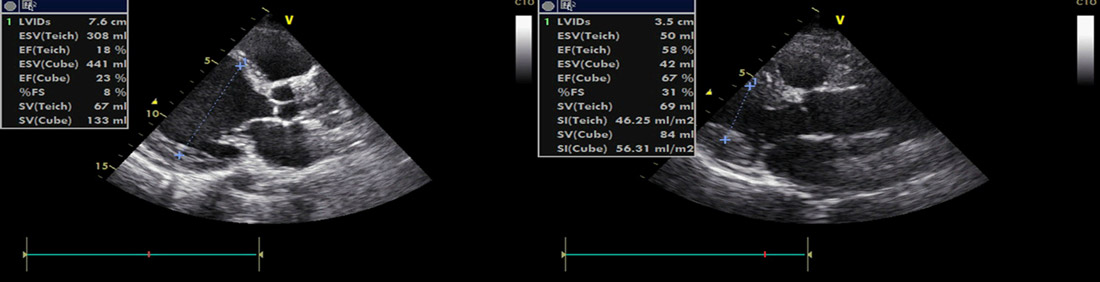
Figure 1:PLAX dimensions; baseline vs 2 yrs post–CRT-P

Figure 2:PLAX dimensions; baseline vs 2 yrs post–CRT-P
• Early consideration of CRT is recommended in patients with reduced EF and LBBB ≥150 ms.
• Device selection should balance clinical benefit and available resources. While CRT-D was optimal, financial constraints necessitated a pragmatic choice of CRT-P, offering substantial symptomatic and benefit on cardiac function, compared to no device.
• In parallel HF therapy should be initiated early and rapidly uptitrated according to tolerance.
The effect of guideline-directed medical therapy (GDMT) on reverse remodelling is attenuated in patients with heart failure with reduced ejection fraction (HFrEF) and left bundle branch block (LBBB) with marked QRS prolongation (≥150 ms). Early consideration of cardiac resynchronization therapy (CRT) is warranted in these patients, as CRT may act synergistically with medical therapy to improve cardiac function, haemodynamics (addressing low cardiac output, hypotension, and bradyarrhythmias) and to facilitate further optimization of heart failure (HF) treatment. This approach is supported by the ESC Guidelines and joint HFA–EHRA–EACVI position papers (doi:10.1002/ejhf.2046; doi:10.1002/ejhf.3150). CRT represents an integral component of HF management in such cases; however, its use remains markedly underutilized. Decisions regarding device implantation should arise from an integrated, multidisciplinary Heart Team discussion involving both HF specialists and electrophysiologists, ideally at the time of diagnosis, to establish a clear and individualized therapeutic strategy. The team of Dr. Mwazo in Kenya deserves to be congratulated.
Regarding GDMT implementation, no therapeutic adjustments were made in this case other than a reduction in diuretics. The use of sacubitril/valsartan at 12.5 mg twice daily is unusually; although manufacturers discourage splitting the lowest available 50 mg tablet, some experts allow 25 mg twice daily as an initial step, followed by titration when possible. Of note, a systolic blood pressure of 90 mmHg should not automatically preclude dose escalation, as therapy initiation does not necessarily lead to further hypotension. Mild dizziness may be tolerated if blood pressure remains stable, since haemodynamic adaptation usually occurs within two weeks. Importantly, diuretics should be adjusted concurrently to avoid hypovolaemia-related hypotension that can limit GDMT optimization. A resting heart rate of 60 bpm may initially restrict beta-blocker uptitration; however, CRT implantation provides protection against bradycardia, enabling gradual dose increases. Dividing the beta-blocker dose into two daily administrations and adjusting every two weeks can further enhance tolerance. In addition, regarding improvement in ejection fraction (HFimpEF), it is difficult to determine whether this primarily resulted from CRT or medical therapy. In any case, continuation of GDMT is strongly recommended in patients with improved EF to maintain therapeutic benefits and prevent relapse. It should be emphasized that GDMT uptitration should be pursued as far as tolerated and should extend well beyond the mere improvement of symptoms.
Finally, while CRT was appropriately employed in this case, it remains essential not to overlook the underlying aetiology of HF, as this is key to guiding personalized treatment. In this context, further investigations, such as cardiac MRI or genetic testing, would have been indicated, although they were not performed due to local resource constraints.
- (doi:10.1002/ejhf.2046; doi.org/10.1002/ejhf.3150)
None
Disclaimer
This case report and/or content does not reflect the opinion of iHF or iheartfunction.com, nor does it engage their responsibility.